
Balancing Chemical Equations Worksheet
1 What is a Chemical Equation? 2 Balancing Chemical Equations Worksheets 3 Why is it Important to Balance the Chemical Equations? 4 Balancing Equations Worksheets with Answers 5 What are Different Types of Chemical Equations? 6 Balancing Equations Practice Worksheet 7 How to Balance a Chemical Equation?

49 Balancing Chemical Equations Worksheets [with Answers] in 2022
2OH - → H 2 O + 1/2 O 2 + 2 e -. This is now balanced, but if you're one of those students that doesn't like fractions in chemical equations, or the question asks for whole number coefficients, just multiply by the denominator ( i.e. double everything): 4OH - → 2H 2 O + O 2 + 4e -.
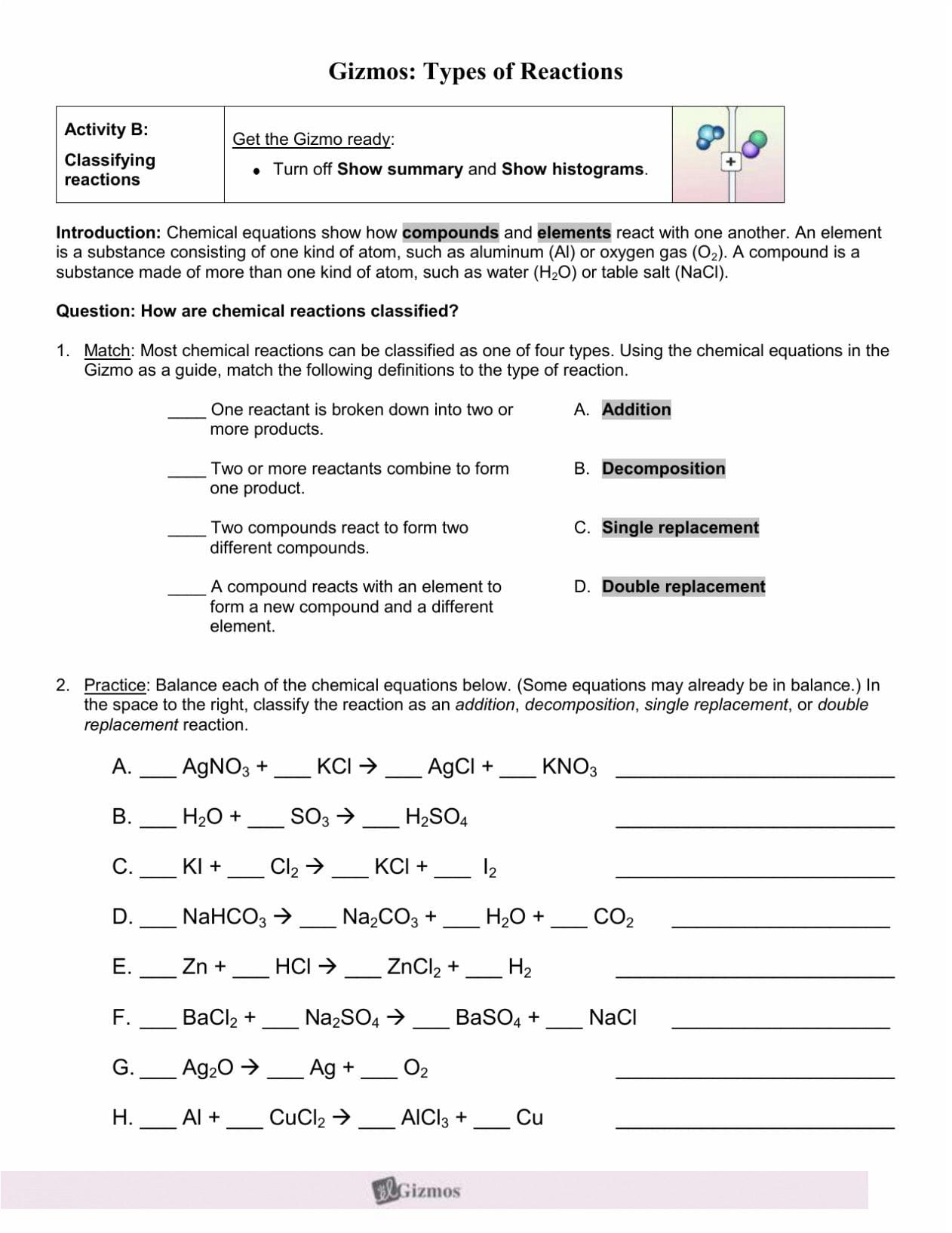
Balancing Chemical Equations Practice Worksheet —
Practice balancing chemical equations. % The current browser window is too small to render this simulation.. Exploration Series | Chemistry Simulations. Worksheet. Aa. Aa. Aa. Aa. Challenge Me. Feedback. FullScreen. Concepts. Tutorial. Assign to Class. Quick Assignments! You can directly assign a simulation to your classes and set a due date.
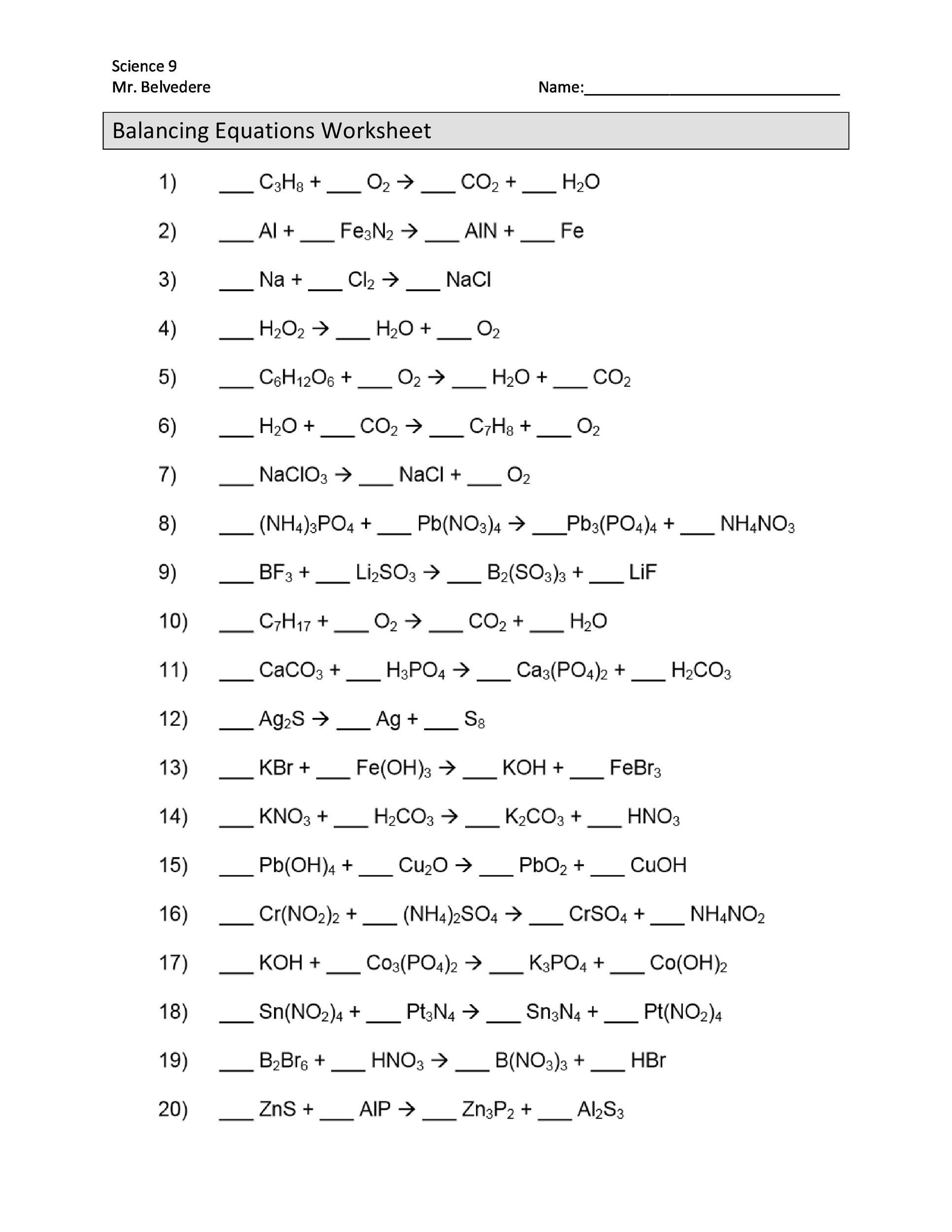
49 Balancing Chemical Equations Worksheets [with Answers]
Write the word equations below as chemical equations and balance: Zinc and lead (II) nitrate react to form zinc nitrate and lead. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Sodium phosphate and calcium chloride react to form calcium phosphate and sodium chloride.

12 Best Images of Balancing Chemical Equations Worksheet PDF
This balancing chemical equations worksheet has ten unbalanced equations to practice your skills. Either right-click and save the image or else download the PDF of the worksheet here. The worksheet prints on a standard sheet of printer paper. Balancing Chemical Equations Worksheet - Answer Key

Identifying And Balancing Chemical Equations Worksheets Answ
Balancing Chemical Equations - Worksheet #1 Balancing Chemical Equations - Answers #1 Balancing Chemical Equations - Worksheet #2 Balancing Chemical Equations - Answers #2 Balancing Chemical Equations - Worksheet #3 Balancing Chemical Equations - Answers #3 Balancing Equations - Worksheet #4 Balancing Equations - Answer Key #4

Balancing equations 2 Unit 6 Balancing Chemical Equations Balance
Directions: First, balance each of the chemical equations below. Then, classify each reaction as synthesis, decomposition, single-replacement, or double-replacement. To earn full credit, write the words out when classifying. Balance the equation. ____ Sb + ____ Cl2 ____SbCl3 ____ Mg + ____O2 ____MgO

Chemistry Balancing Chemical Equations Practice Worksheet Answer Key
This Balance Chemical Equations practice sheet is useful to help students balance chemical equations. This ten equation worksheet is available in PDF format. The answer key is also available in PDF format or if you'd prefer a quick look, an image of the completed sheet can be found here. Check out our other Balancing Chemical Equation Worksheets.

Chemistry Balancing Equations Practice Worksheet Answer Key / Balancing
The ultimate goal for balancing chemical equations is to make both sides of the reaction, the reactants and the products, equal in the number of atoms per element. This stems from the universal law of the conservation of mass, which states that matter can neither be created nor destroyed.
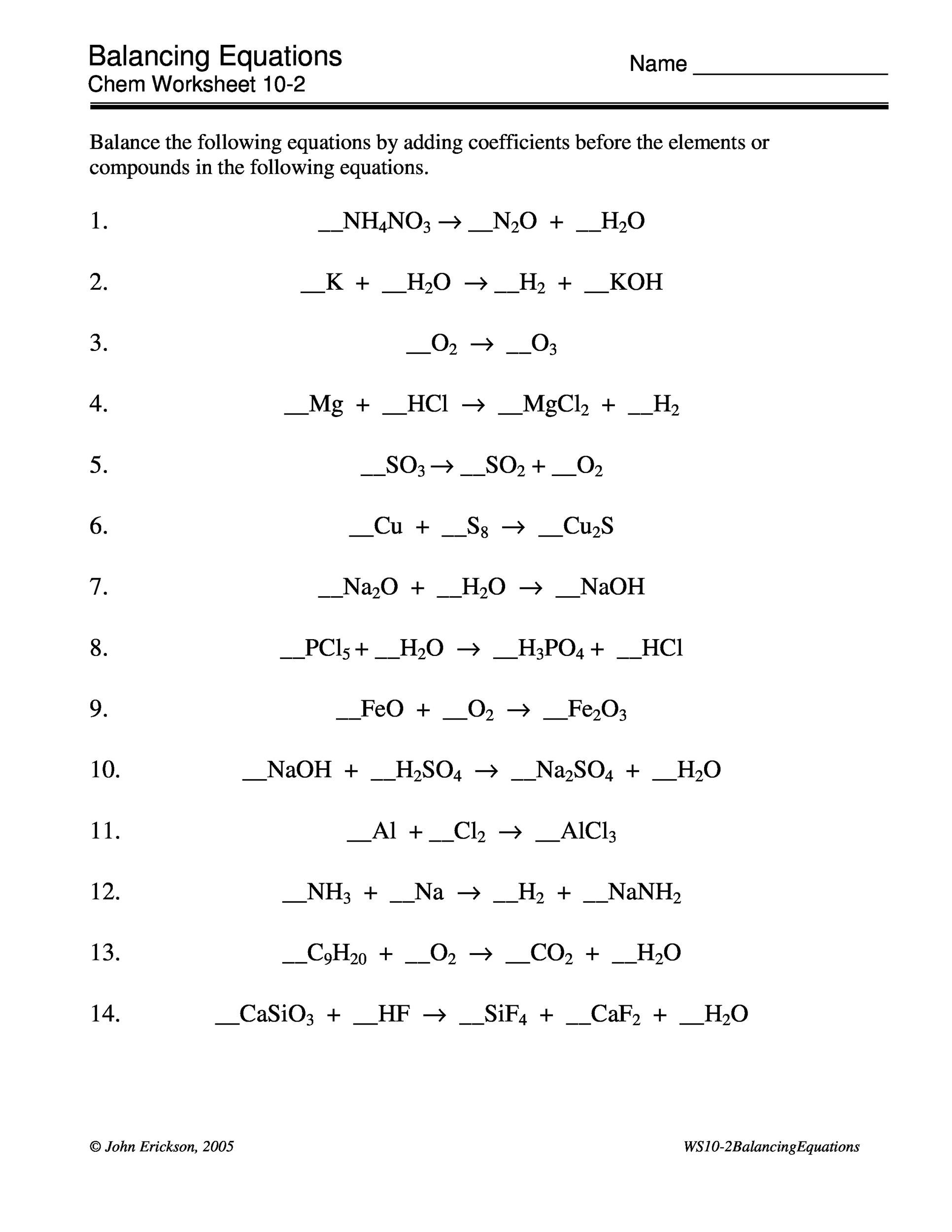
49 Balancing Chemical Equations Worksheets [with Answers]
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.

Balancing Chemical Equations Worksheet Pdf —
Balancing Chemical Equations - Answer Key. Balance the equations below: N2 + 3 H2 Æ 2 NH3. KClO3 Æ 2 KCl + 3 O2. 2 NaCl + 1 F2 Æ 2 NaF + 1 Cl2. 2 H2 + 1 O2 Æ 2 H2O. Pb(OH)2 + 2 HCl Æ 2 H2O + 1 PbCl2. AlBr3 + 3 K2SO4 Æ 6 KBr + 1 Al2(SO4)3. CH4 + 2 O2 Æ 1 CO2 + 2 H2O.

Balancing Chemical Equations Worksheet
Balancing chemical equations 1 Google Classroom Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O Note: All reactants and products require a coefficient of at least one. Stuck? Review related articles/videos or use a hint. Report a problem Do 4 problems

49 Balancing Chemical Equations Worksheets [with Answers]
PROBLEM 5.1.1. 4. Write a balanced equation describing each of the following chemical reactions. Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6.

49 Balancing Chemical Equations Worksheets [with Answers]
Balancing chemical equations (KS3/GCSE) - Questions © www.chemistrytutor.me 2018 Page 1 of 3 1. _O 2 + _NH 3-> _HNO 3 + _H 2 O 2. _O 2-> _O 3 3. _H

Chemistry Balancing Chemical Equations Worksheet 3 Answer Key
Balancing chemical equations requires practice. Once you've done it a few times, it becomes easier and easier. This balancing chemical equations practice sheet has ten more unbalanced chemical equations to solve. Download a PDF of this worksheet here. A PDF of the answer key is also available here.

Answer Key Balancing Chemical Equations Practice Worksheet With Answers
in CuSO4 has an oxidation number of +6 So between 3CuS to 3 CuSO4 there will be a total increase of 3 x +8 = +24. This means N must decrease by -24 In HNO3 the oxidation number Of N is +5 and in NO it is +2. The change is -3 Therefore there must be 8 N's to decrease by -24 (8 x-3). So we must have 8HNO3 and 8NO.